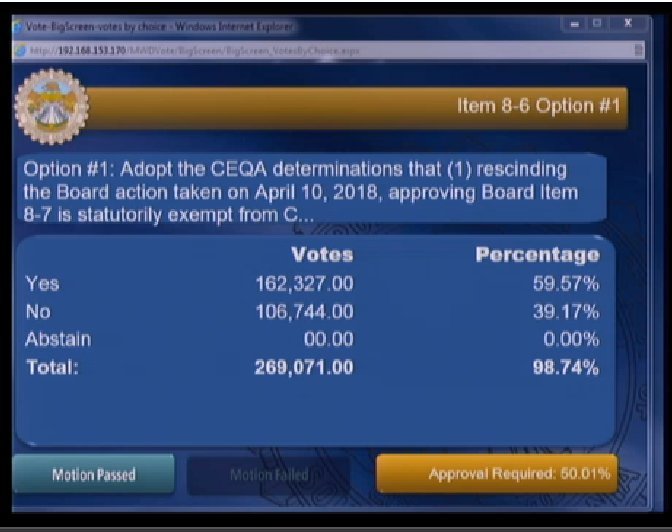
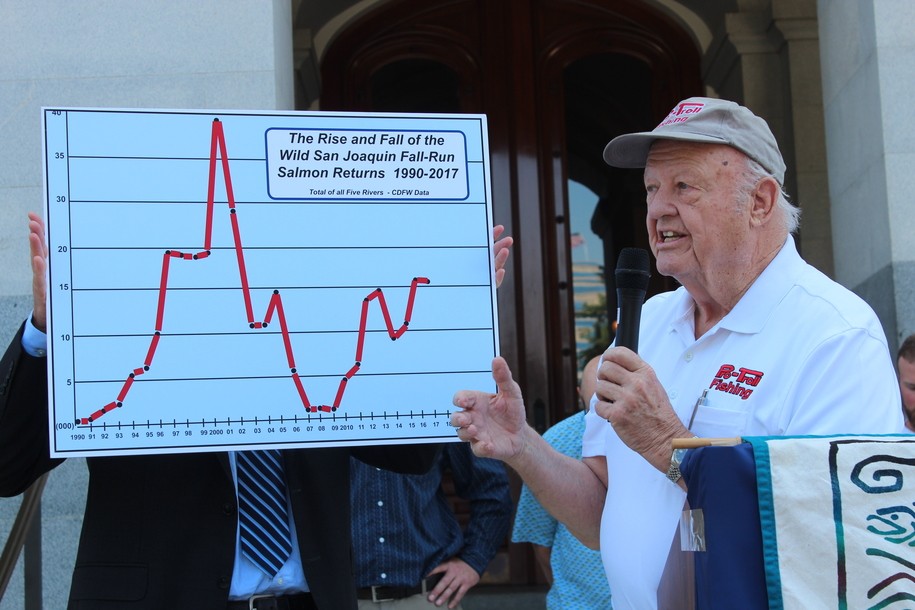
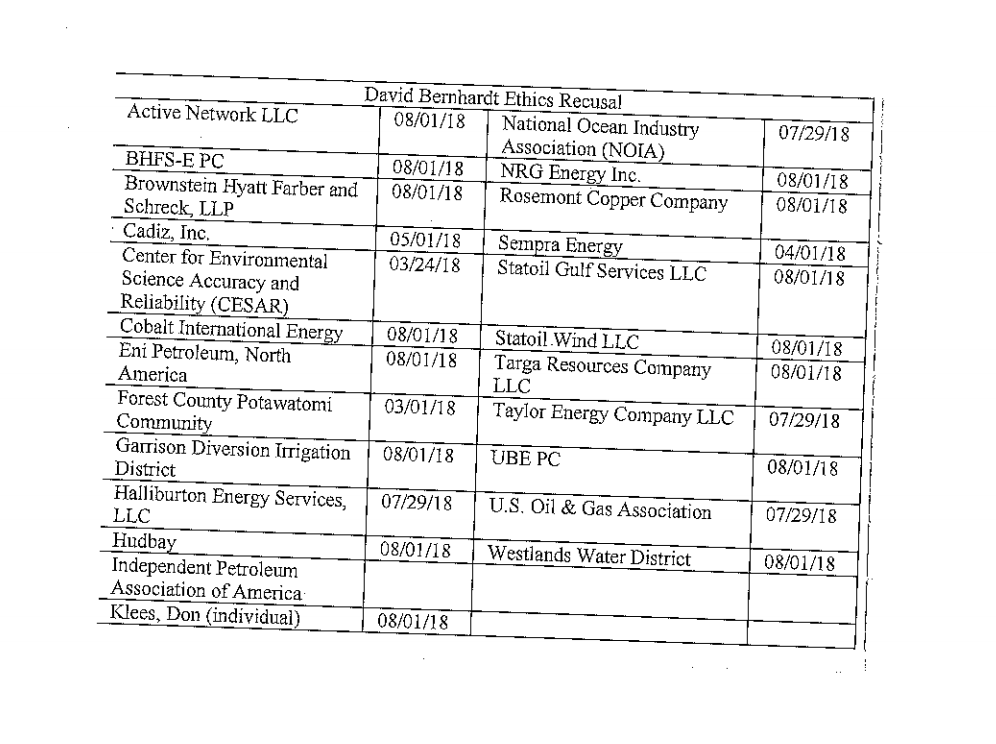
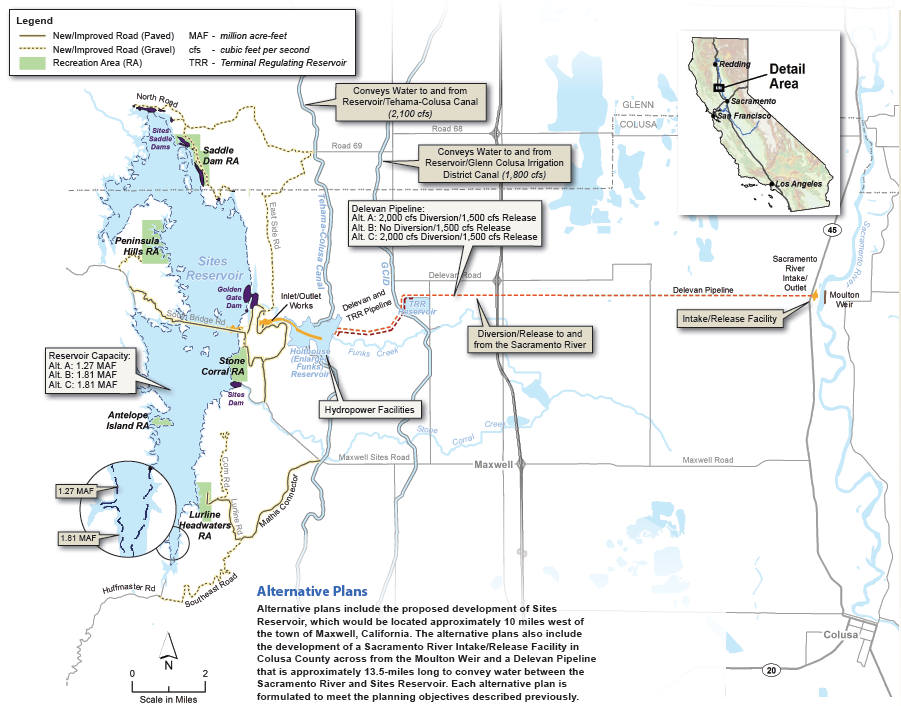
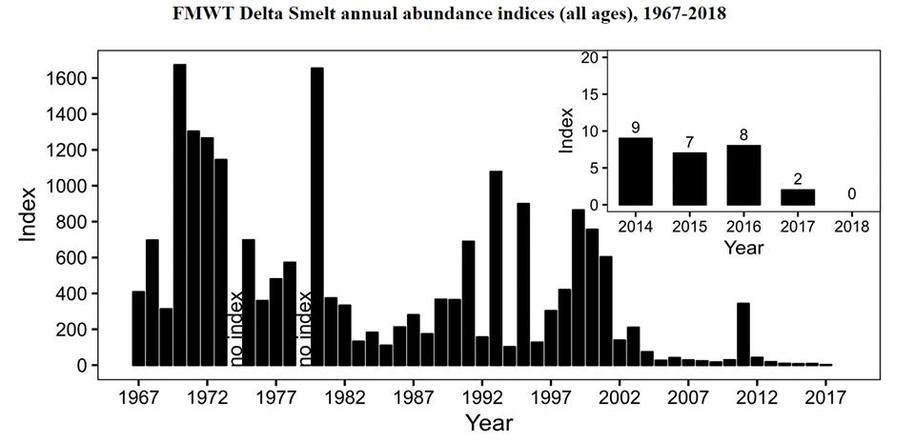
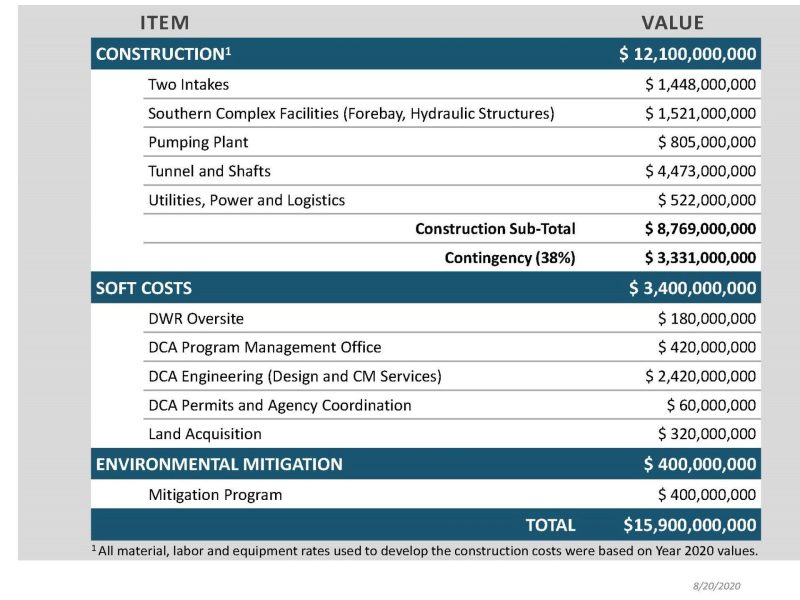
The federal Food and Drug Administration (FDA) on March 8 announced that it will lift the import ban on eggs of AquaBounty's genetically modified salmon product, drawing praise from the company and strong criticism from fishing organizations.
“Today, we are taking another important step by deactivating a 2016 import alert that prevented GE salmon from entering the U.S.” claimed FDA Commissioner Scott Gottlieb, M.D. “The FDA’s approval of the application related to AquAdvantage Salmon followed a comprehensive analysis of the scientific evidence, which determined that the GE Atlantic salmon met the statutory requirements for safety and effectiveness under the Federal Food, Drug, and Cosmetic Act.”
AquaBounty Technologies, Inc. said the lifting of the import alert would allow the Company to start farming AquAdvantage Salmon in Indiana.
“We are delighted that FDA has lifted the import alert, which will allow AquaBounty to begin producing and marketing AquAdvantage Salmon in the United States,” said Sylvia Wulf, Chief Executive Officer of AquaBounty. “As FDA notes in this announcement, our salmon was approved by the agency over three years ago based upon a very comprehensive science-based review process, which established that our salmon was safe, nutritious, and environmentally sound and met all other regulatory requirements.”
“We will immediately start the process to import AquAdvantage eggs from our hatchery in Canada to begin grow out at our Indiana facility,” she said.
Both the Pacific Coast Federation of Fishermen’s Association and the Golden Gate Salmon Association blasted the decision and vowed they will fight to keep fighting against the import of frankenfish for the U.S. market.
"A loophole has now been created that will allow the first genetically modified animal engineered for human consumption to enter the US market: GMO Atlantic salmon,” said Pacific.






































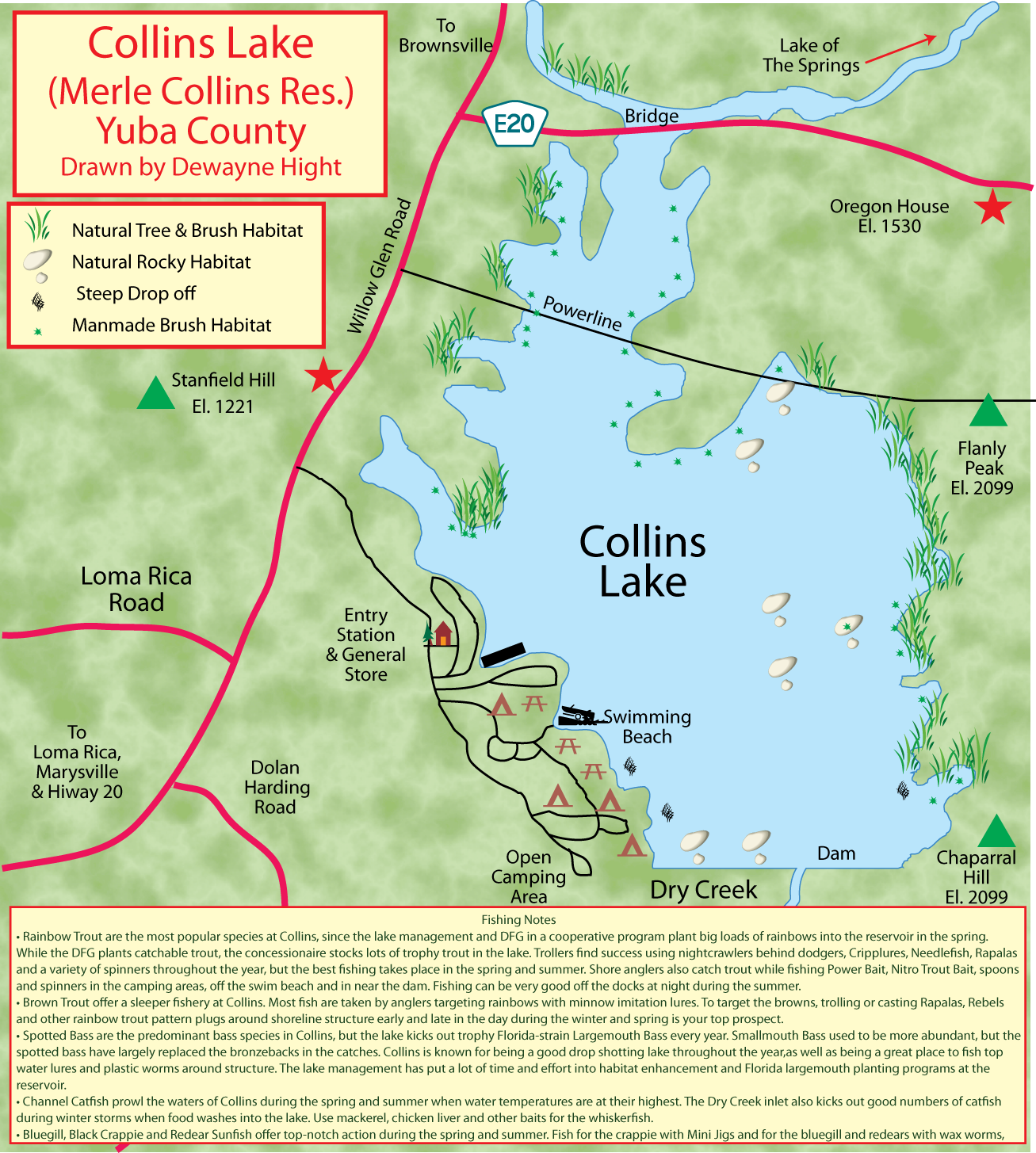
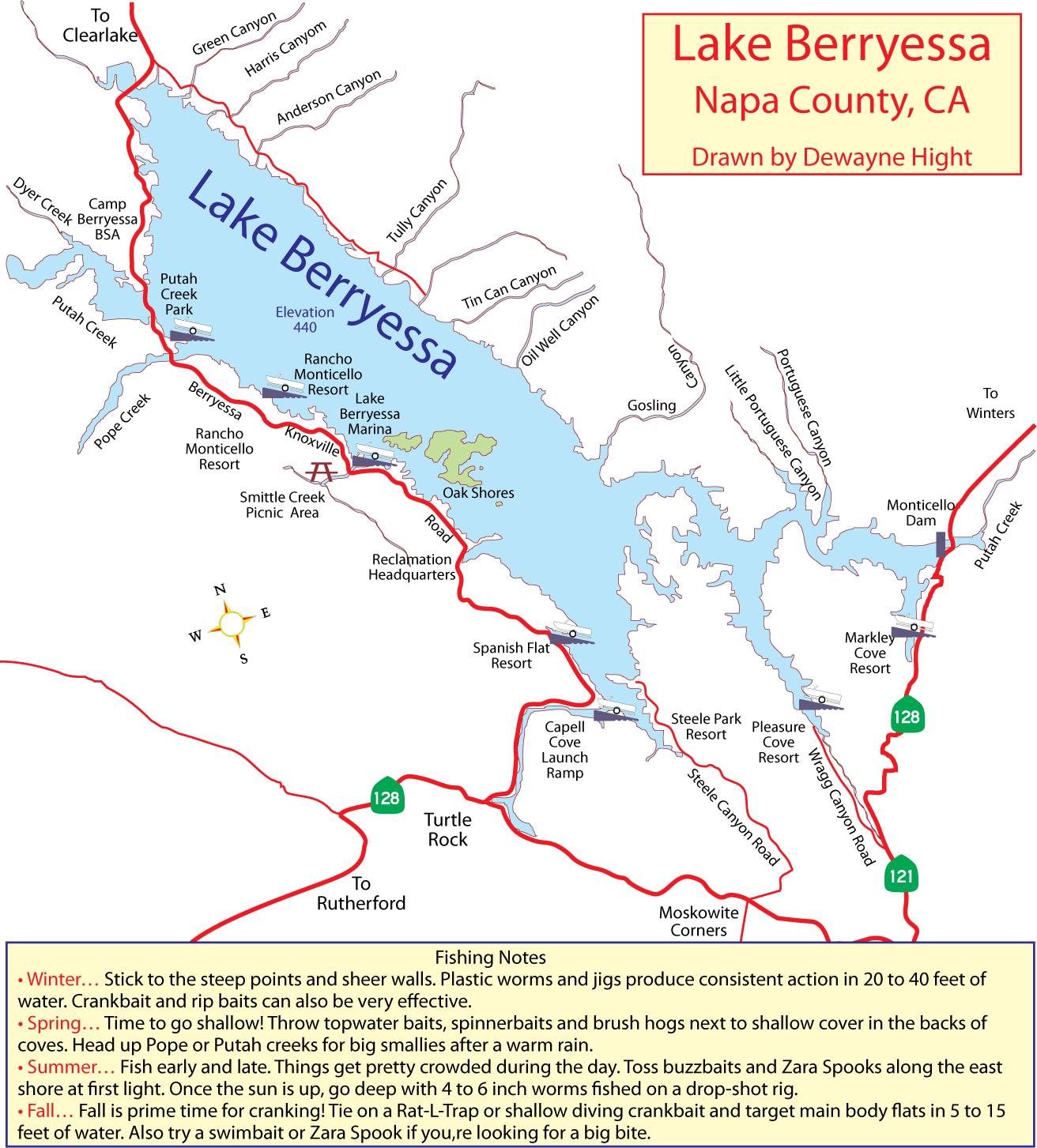




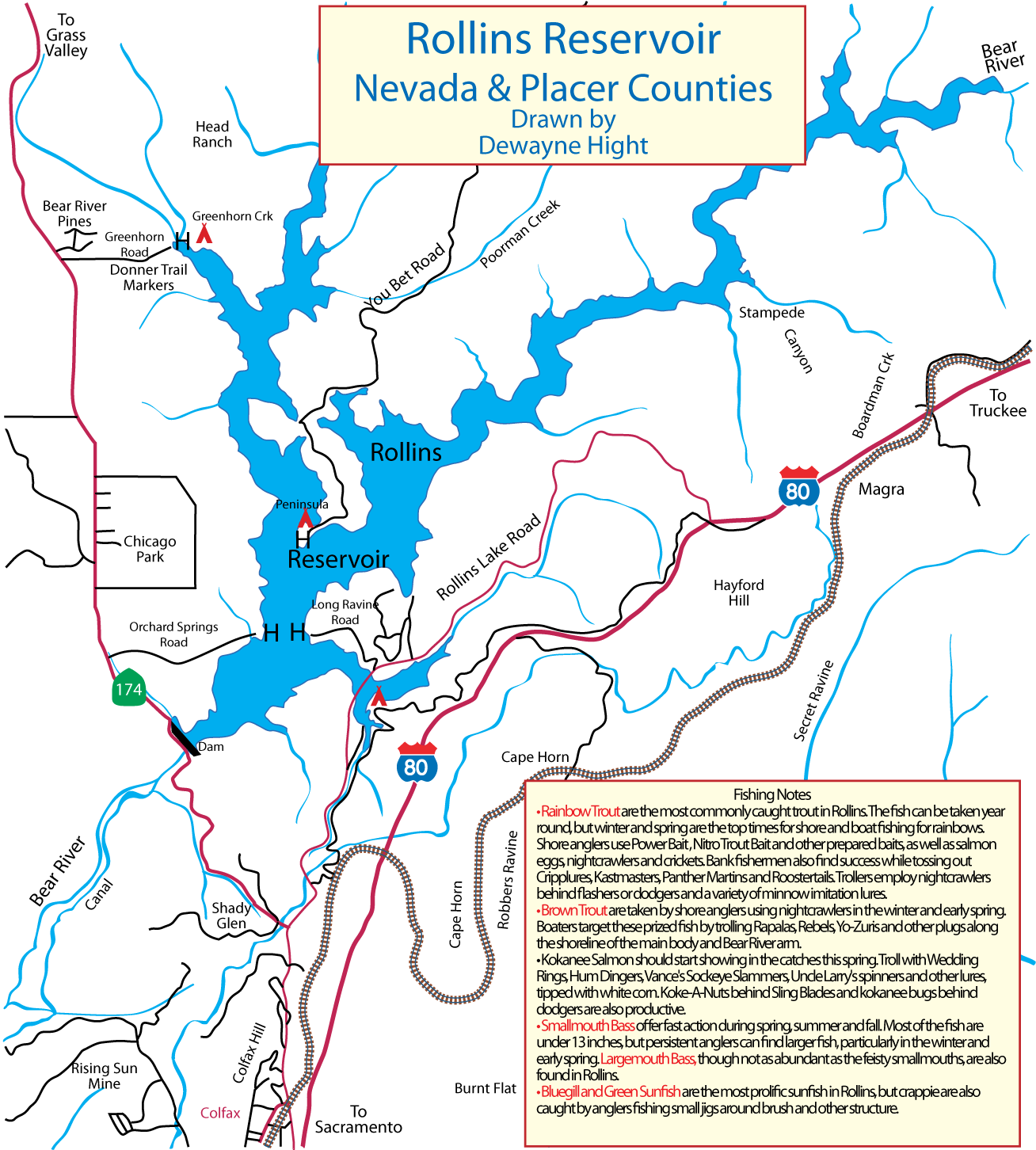

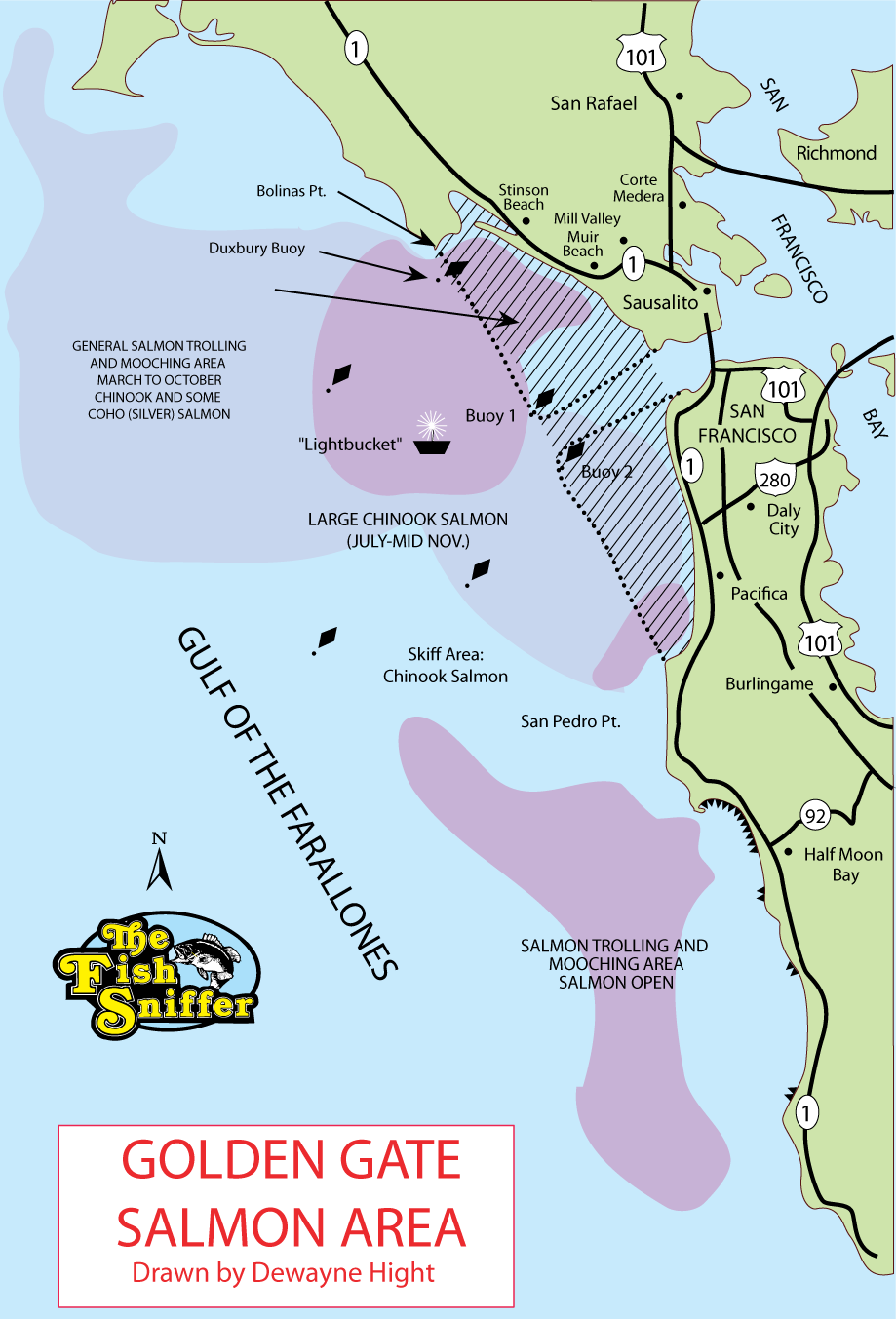






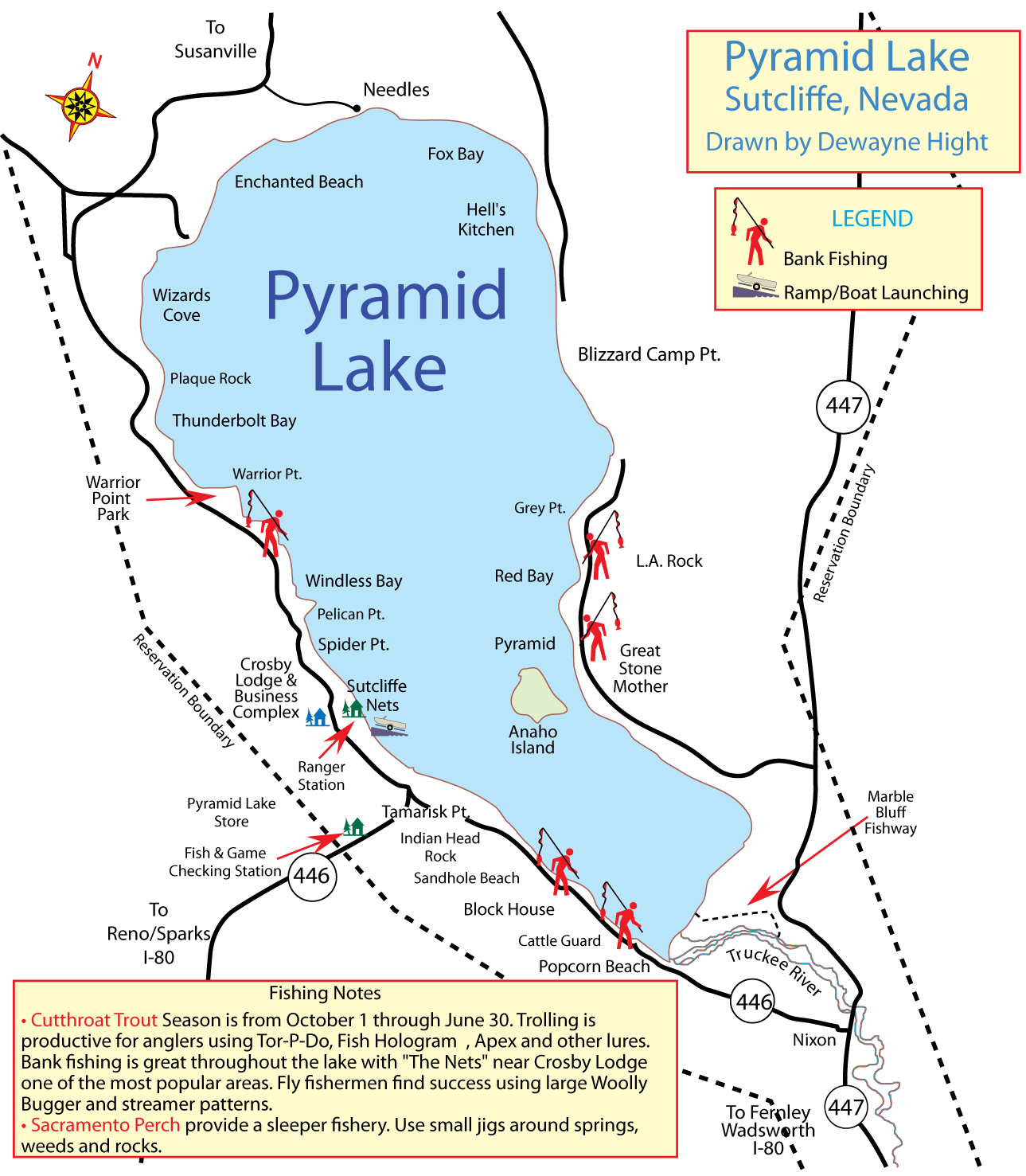





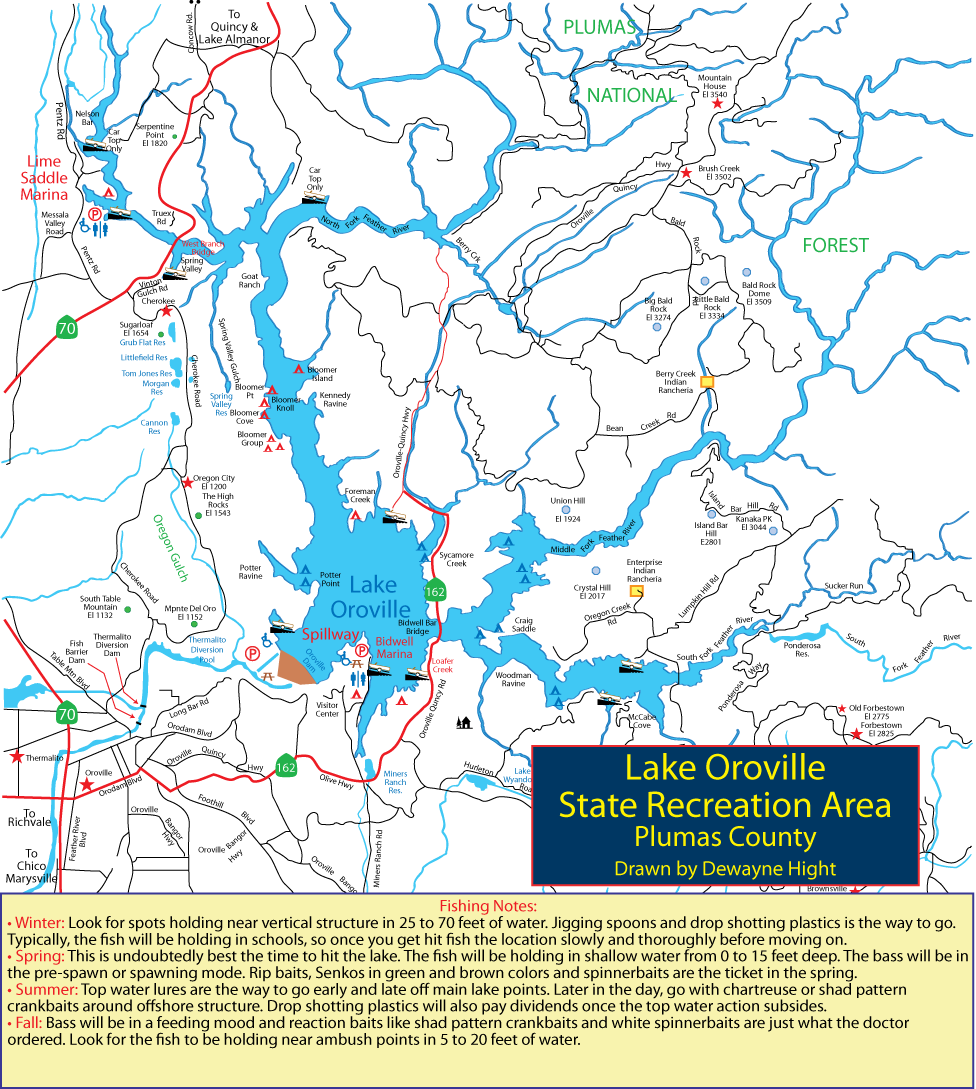

















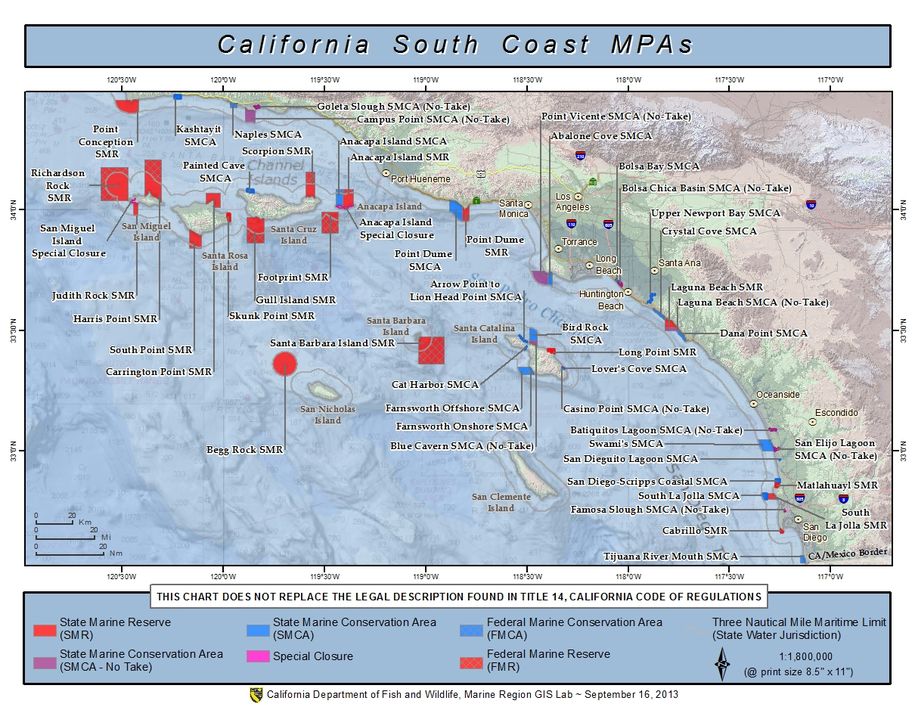
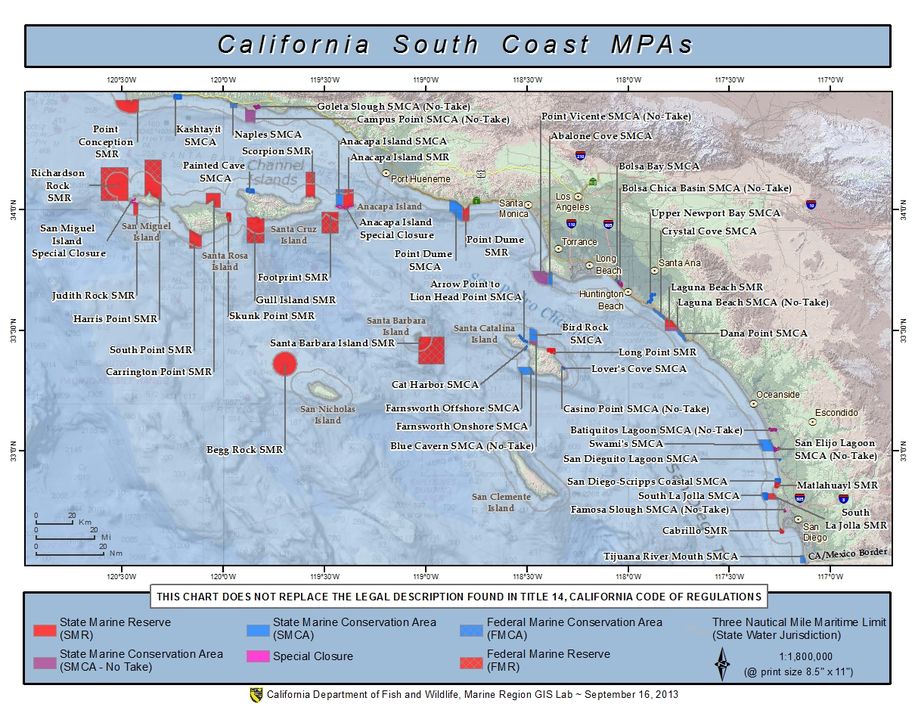
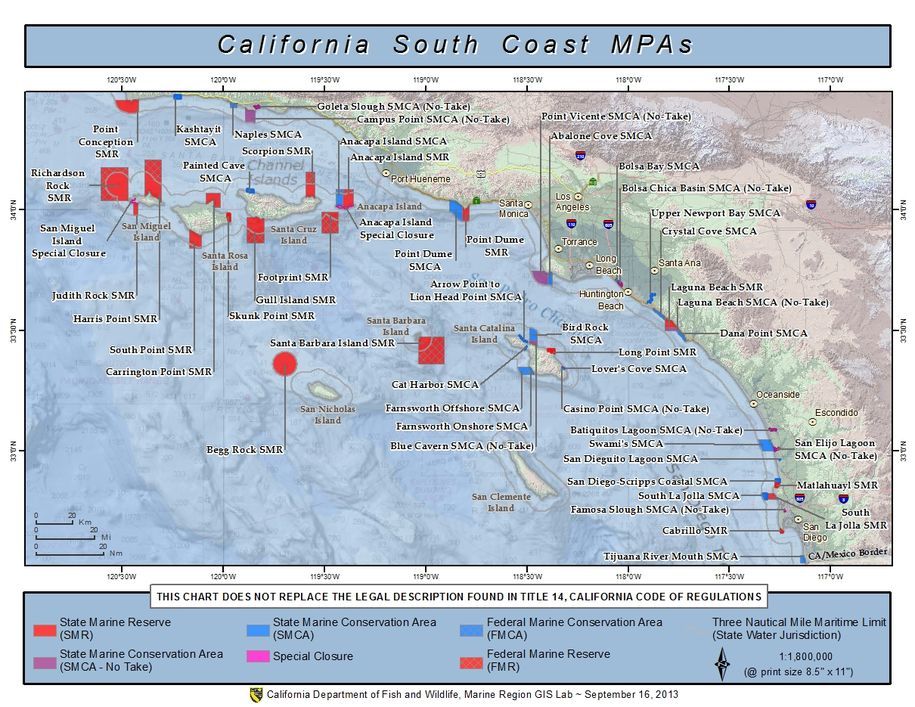
 (Berkeley) The end of the rockfish season is seen by many as the end of the saltwater fishing season. This year the season ended on a somber note with crab fishing closed and rough seas that kept both charter and private boats in port.
(Berkeley) The end of the rockfish season is seen by many as the end of the saltwater fishing season. This year the season ended on a somber note with crab fishing closed and rough seas that kept both charter and private boats in port.